In-situ Workflow
In-situ lamella preparation workflow starts at sample preparation and terminates with high-resolution tomographic data acquisition on a high-end TEM such as Krios. However there are multiple entry points to any in-situ project depending on the biological question.
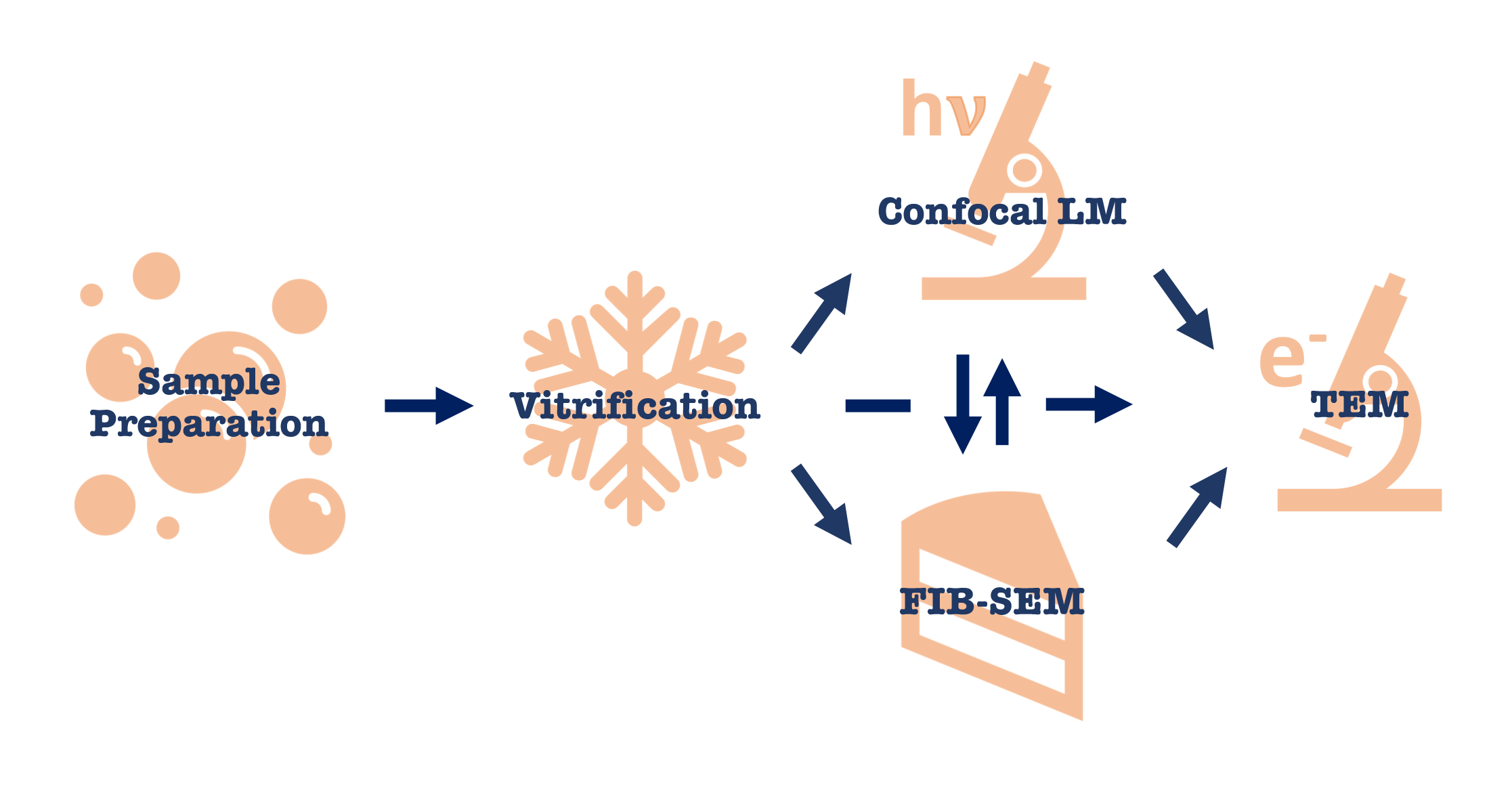
Sample preparation step is different for cells, tissues and expression systems. For thinner specimens (i.e. bacterial cells, yeast and cell lysates) vitrification can be done by a simple plunge-freezing into liquid ethane. For thicker specimens (i.e. mammalian cells, cell aggregates and tissues) high-pressure freezing is needed.
After vitrification there are several options available: light microscopy to verify sample application and fluorescent signal needed for CLEM; FIB-SEM milling for preparation of thinned samples or contrast imaging; direct data acquisition on TEM if the sample is thin or has thin areas. If fluorescent signal is present, thinned specimens after FIB-milling can be verified to still have the signal on a light microscope. Some samples can bypass light microscopy and FIB-SEM step altogether and data can be acquired in TEM on naturally thin areas such as cell edges.

