Advancing the application of cryoelectron tomography of intact cells and tissues.
We perform standard cryoelectron tomography (cryoET) sample preparation, provide access to advanced cryoET specimen preparation capabilities, train users in the development of the skills needed for independent research in cryoET, and continually update methods and technologies to stay at the forefront of this field.
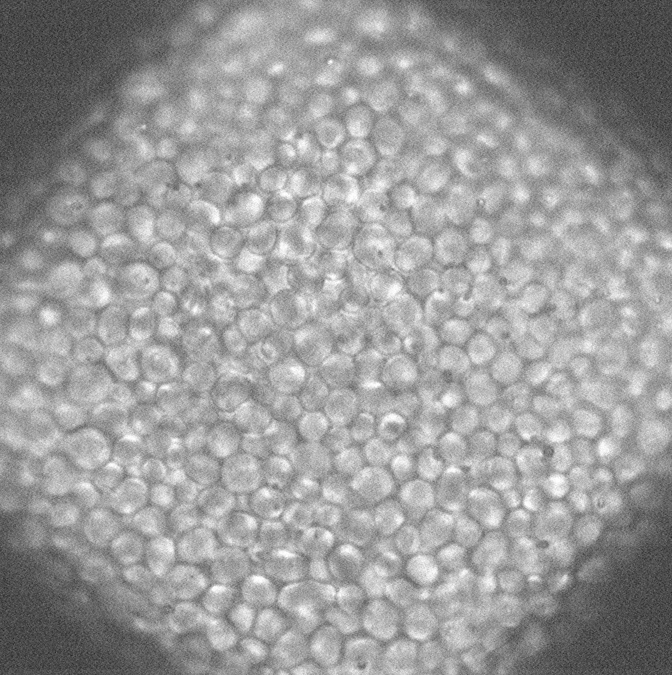
Light Microscopy
Light microscopy is done using Zeiss LSM 900 equipped with Airyscan2 detector and Linkam cryostage
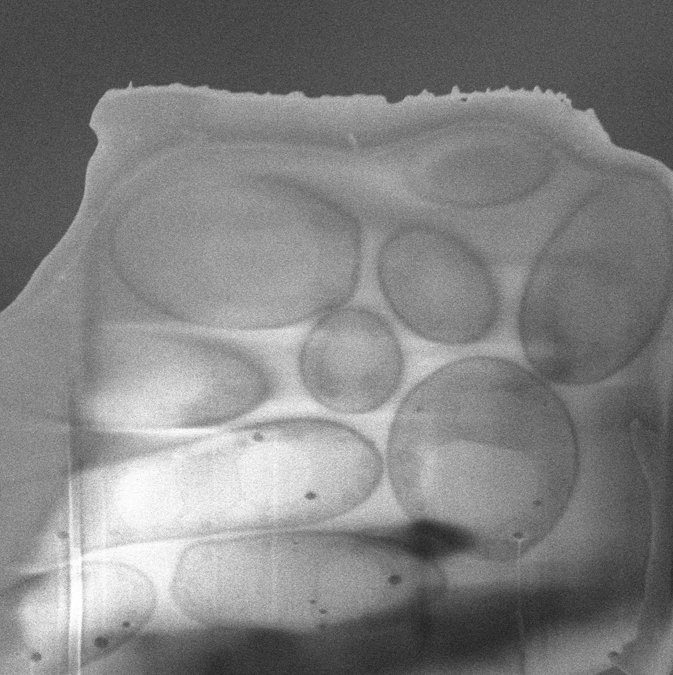
Focused Ion Beam Scanning Electron Microscopy
Lamella thinning and other FIB needs are accommodated by our Aquilos 2 Cryo FIB-SEM
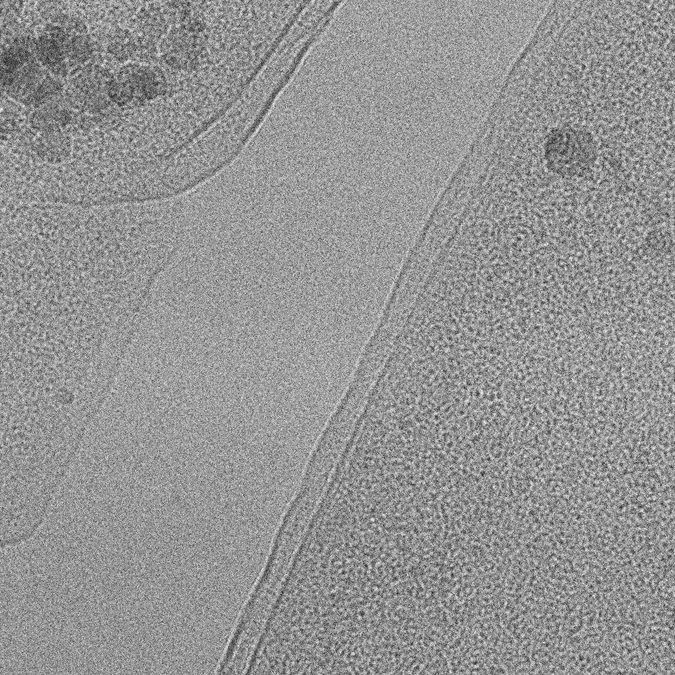
Transmission Electron Microscopy
Data from thin specimens will be collected on a energy-filtered Krios TEM
WORKFLOW
Every in-situ workflow is as unique as the biology in question. It usually starts at sample preparation and terminates with a high-resolution cryoET data collection
Specimen preparation
The ideal specimen for focused ion beam (FIB) thinning is a cell or a group of cells positioned on an EM-grid. However, different questions require different approaches. Bulk tissue, cell suspension and bacterial colonies are all candidates for in-situ analysis. While most of the specimen preparation is expected to be done by the user, we will aid and provide expertise when necessary.

Vitrification
Vitrification of biological specimens is achieved by either plunge-freezing into liquid ethane or by high-pressure freezing (HPF). HPF is recommended for tissue samples, large cell aggregates and other bulk specimen, while conventional plunge-freezing is used for smaller cells, like bacteria and yeast, and individual eukaryotic cells.

Light microscopy
Frozen, fluorescently labeled cells can be first analyzed in a dedicated confocal microscope equipped with a cryo stage. Specimens can be analyzed to ensure that the expected phenotype is observed, ice is sufficiently thin and the grids themselves have enough targets for downstream analysis.
FIB-SEM
Vitrified specimens can be FIB-milled to a required thickness to allow data collection in a Transmission Electron Microscope (TEM). Alternatively, volume data can be acquired during the milling process yielding 3D reconstructions on a whole-cell scale.
TEM
Once samples are done here, they will be shipped to the central hub or back to a user, where final imaging experiments, usually tomographic data collection, are conducted.
APPLICATION PROCESS
Application to the NCITU is administered through the joint tomography center portal.
Project meeting
Prior to submitting an application, the in-situ team would meet with you to discuss your project and assess its feasibility. We would be able to provide suggestions and expertise in order for you to get the most out of our center.

Application
A centralized application process will make submitting a project simple: a one-page project description will be submitted through a central hub, peer-reviewed and assigned to a service center. Application process is currently in active development while we are booting up operations.

Review
When you submit your application, it will be reviewed by a committee which will decide if it is approved based on given scores within the review categories. If your application is approved, it will be added to a queue and time will be allocated accordingly.

Project execution
Once your project is allocated a timeslot, you will be able to send us your samples. We will store your samples until your session start, where we will begin with cell growth, vitrification or milling based on your project.

